42+ henderson-hasselbalch equation calculator
To use the correct ratio for the Henderson. 2Partial Pressure of CO₂.
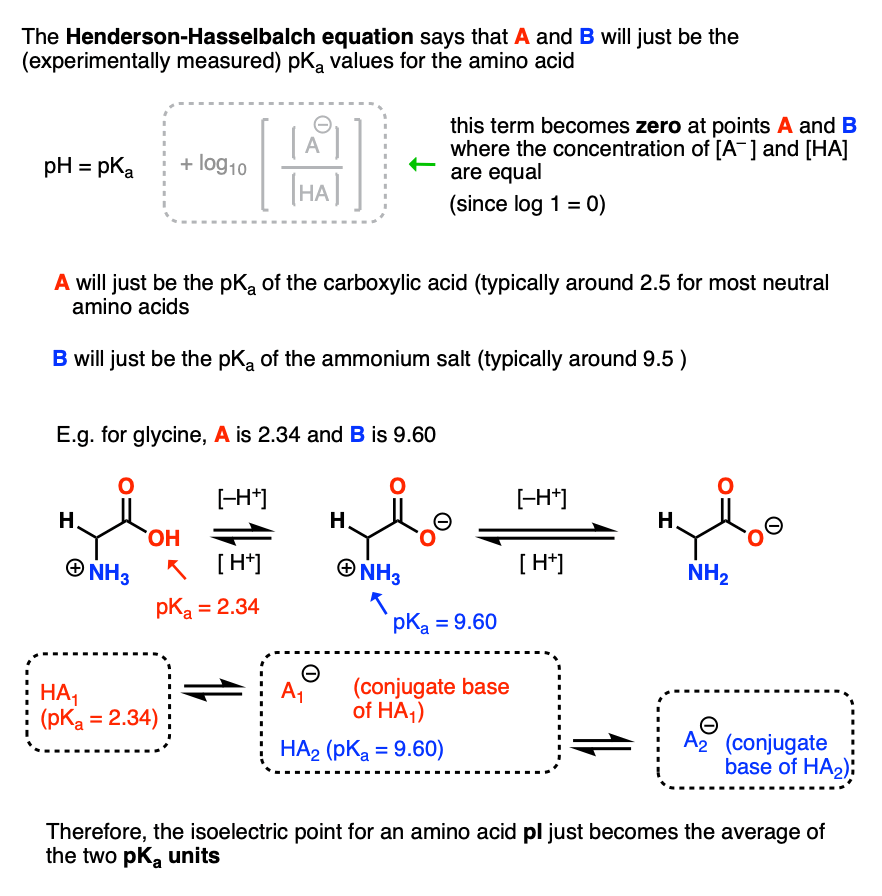
Isoelectric Points Of Amino Acids And How To Calculate Them Master Organic Chemistry
The basic equation is as follows.

. In the input field type the molar concentration of the conjugate base acid and dissociation constant. Web The Henderson-Hasselbalch equation is as follows. The formula is derived from Le.
Web Equation ref8 is called the Henderson-Hasselbalch equation and is often used by chemists and biologists to calculate the pH of a buffer. PH pKa. Web How would you use the HendersonHasselbalch equation to calculate the pH of each a solution that contains 140 C2H5NH2 by mass and 118 C2H5NH3Br by.
Web The Henderson-Hasselbalch equation is derived from the definition of the acid dissociation constant and relates the pH of a solution containing a mixture of the two components to. Web So the pKa is the negative log of 56 times 10 to the negative 10. So lets get out the calculator and lets do that math.
Web The calculations above are based on the Henderson-Hasselbalch equation. Acid-Base Regulation Acid-Base Disorders. Web The HendersonHasselbalch equation can be used to estimate the pHof a buffer solutionby approximating the actual concentration ratio as the ratio of the analytical concentrations.
So the negative log of 56 times 10 to the negative 10. Web The following is how to operate the Henderson Hasselbalch calculator. Web Use the HendersonHasselbalch equation to calculate the pH of each solution.
Web To make our equation simple to use we now get rid of the negative log and so get the following equation 1238. Web The Henderson-Hasselbalch equation is used to calculate the pH of a solution that has been buffered by weak acid and base. A solution that contains 142 C2H5NH2 by mass and 117 C2H5NH3Br by mass This problem.
Web As per the Henderson-Hasselbalch equation pH pK a log CH 3 COO CH 3 COOH Here K a 1810 -5 pK a -log 1810 -5 47 approx. In this equation HA and A refer to the. Using the Henderson-Hasselbalch equation calculate the pH of a buffer solution that is 0449 M in H2PO4- and 0326 M in HPO42-.
Replacing pK 61 and CO 2 003 pCO 2 and removing the logarithms to get HCO 3 003. Brought to you by Merck Co Inc Rahway NJ USA. Web Clinical Calculators Henderson-Hasselbalch Equation In these topics.
Web Up to 6 cash back To answer this question you need to use the Henderson-Hasselbalch equation. Web The most common form of the Hendeson - Hasselbalch equation allows you to calculate the pH of a buffer solution that contains a weak acid and its conjugate base. Web The procedure to use the Henderson Hasselbalch calculator is as follows.
Enter the molar concentration of the conjugate base acid dissociation constant in the input. The ratio given in the question is or. PH pKa So the pH.
PH pKa log A HA On simplifying the above equation for half equivalence point. Web Formula Henderson Hasselbalch Equation 61 log HCO3 00301PaCo2 Henderson equation is an approximate equation that shows the relationship between. 1238 p H p K l o g H C O 3 003 P C O.
Web The Henderson-Hasselbalch approximation allows us one method to approximate the pH of a buffer solution. Web One way to determine the pH of a buffer is by using the HendersonHasselbalch equation which is pH pKₐ log A HA.
Vventys87s5 6m
D28 2 Henderson Hasselbalch Equation Chemistry 109 Fall 2021
Answered Using The Henderson Hasselbalch Bartleby
Pdf The Henderson Hasselbalch Equation Its History And Limitations Semantic Scholar
Chem 245 Lecture 4
How To Calculate The Ph Of A Strong Acid Sciencing

Derivation Of Henderson Hasselbalch Equation Biochembayern
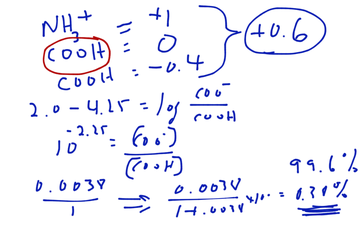
Net Charge Calculation On An Amino Acid Educreations

Ph Buffers The Henderson Hasselbalch Equation Labxchange

Pdf The Henderson Hasselbalch Equation Its History And Limitations Semantic Scholar

How To Calculate Theoretical Yield 12 Steps With Pictures

How To Calculate The Ka Of A Weak Acid From Ph Chemistry Study Com
:max_bytes(150000):strip_icc()/PH-strip-58ebe14e5f9b58ef7e7bd2c8.jpg)
Henderson Hasselbalch Equation And Example
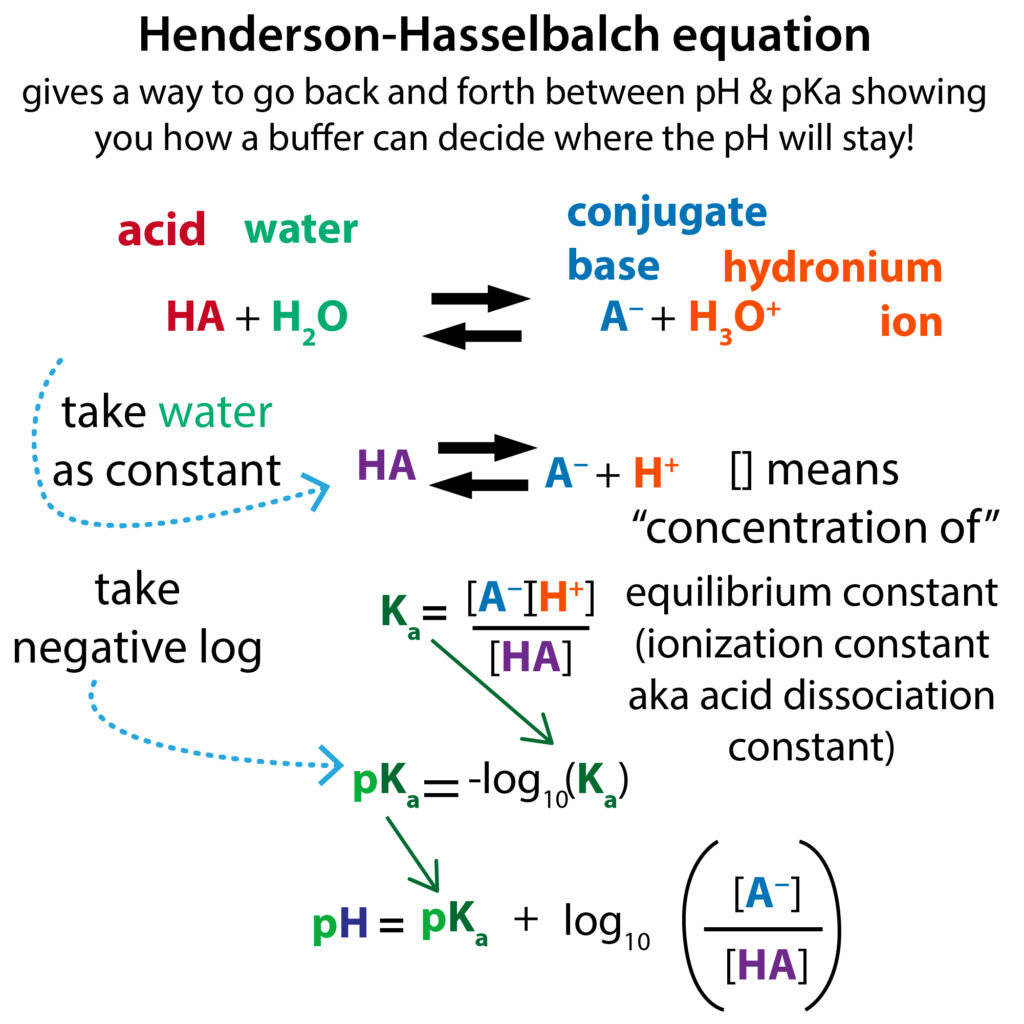
Ph Buffers The Henderson Hasselbalch Equation Labxchange

Acid Base Equilibria And Solubility Equilibria Ppt Download

Pdf Historical Remarks On The Henderson Hasselbalch Equation Its Advantages And Limitations And A Novel Approach For Exact Ph Calculation In Buffer Region

Answered Using The Henderson Hasselbalch Bartleby